Stereochemical notations
Stereo configurations of molecules in the mcule database are standardized and displayed at mcule.com accordingly. Here we give a description of our drawing standards, which are necessary to interpret stereo configurations in the mcule database correctly.
The mcule database currently supports the representation of the two most common forms of stereoisomerism: optical isomerism and cis-trans isomerism. Configuration of optical isomers is usually specified and displayed using special bond types at the tetrahedral centers, whereas cis-trans configurations are primarily represented by 2D geometry.
Cis-trans configuration
Ligand geometry around double bonds defines cis-trans configurations without any special marking. Ligands with ‘trans’ positions and ‘cis’ positions denote the corresponding E or Z configurations. If the configuration is not known, the double bond is replaced with a crossed double bond.
The mcule registration system supports the distinction between unknown and unspecified/undefined cis-trans configurations. Unknown cis-trans configuration represents a structure having either E or Z configuration but definitely one of them. In unspecified/undefined cases the structure can also represent the mixture of the cis and trans isomers.
Although the mcule registration system can distinguish between unknown and unspecified/undefined cases, the currently implemented molecule editor and visualization tools (ChemWriter and ChemVector) don’t provide different bond types for these two configuration types. Unknown and unspecified/undefined cis-trans configurations are therefore both displayed by crossed double bond.
Tetrahedral configuration
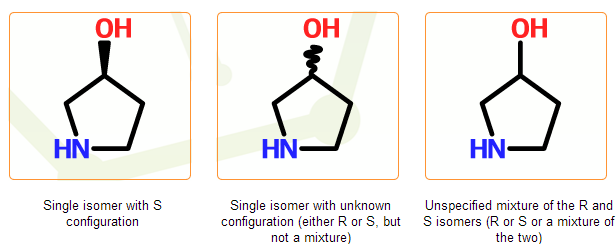
Geometry around a tetrahedral center is specified using wedge bonds pointing to the center with their narrow ends. Up (solid) and down (hashed) wedge bonds denote well-defined geometry. Wavy bond is used to denote centers with unknown geometry: in this case the configuration is either R or S, but definitely one of them. Unmarked centers (without wedge or wavy bonds) denote fully unspecified geometry, the configuration can be R or S or a mixture of the two.
Stereo configuration types
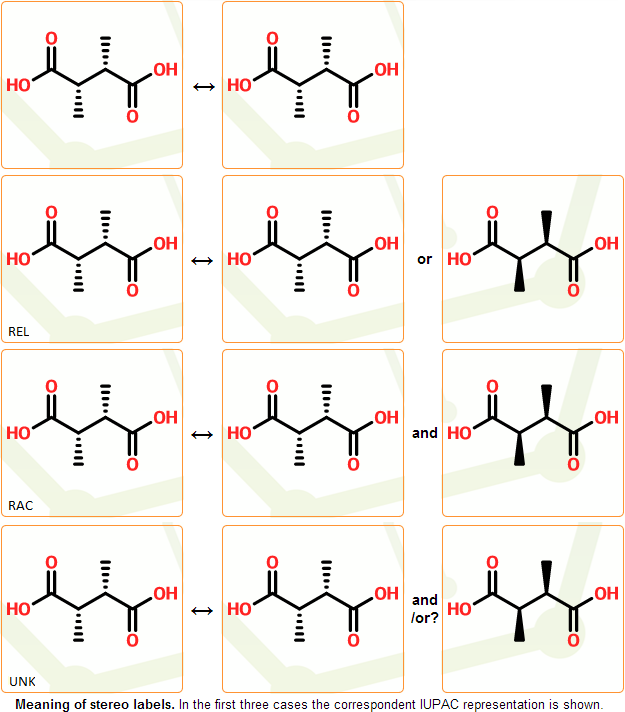
In order to simply denote more complicated and less specified structures, four stereo configuration types are introduced: absolute, relative, racemic and unknown.
| stereo configuration type | meaning |
|---|---|
| absolute | the shown isomer is present |
| relative | the shown isomer or its enantiomer is present |
| racemic | the shown isomer and its enantiomer is present in 1:1 mixture |
| unknown | configuration type is uncertain: probably absolute but can be relative or racemic |
Interpretation of the absolute, relative and racemic types is shown in table. The unknown configuration type indicates that configuration type cannot be the determined surely. One should treat structures with unknown configurations as if they have absolute configuration not confirmed by the compound supplier.
Stereo configuration types are indicated by the displayed REL, RAC, or UNK stereo labels. If the structure has absolute stereo configuration type or does not contain any stereocenters, no stereo labels are displayed. In these cases the interpretation of the configuration is straightforward.