Structure specification levels
In the mcule database chemical structures can be described in three structure specification levels: conformers, structures and compounds.
Conformers have a fully specified 3D structure including the position of all hydrogens. They are in a fixed tautomeric and protonation state, and they have a known absolute stereo configuration (as reflected in the geometry).
Structures refer to molecular structures representing a fixed tautomeric and protonation state as reflected by the 2D structural formula. They can have a fully defined or a partially or fully unknown/undefined stereo configuration.
Compounds refer to chemical compounds that can be represented by multiple molecular structures, i.e. with multiple tautomeric and protonation forms. Their stereo configuration can be well-defined as well as fully or partially unknown / undefined, similarly to the case of structures.
| Structure specification level | Identical | Different |
|---|---|---|
| Compound | Mesomers, tautomers, protonation states, conformers | Stereoisomers |
| Structure | Mesomers, conformers | Stereoisomers, tautomers, protonation states |
| Conformer | Mesomers | Stereoisomers, tautomers, protonation states, conformers |
Chemical structures with different stereo configurations are distinguished at all specification levels. Depending on the stereo configuration type, they represent a single isomer or a set of potential isomers and/or mixtures of isomers. Their stereo configurations are treated identical if and only if the represented isomers / set of isomers are identical. If the configuration differs, the chemical structures are treated as different database entries.
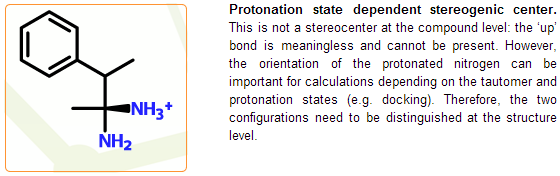
Compounds can have more symmetries than structures. In compounds neither the number of hydrogens nor the position of some hydrogens are fixed. A compound can represent all realistic tautomeric and protonation states (structures). Therefore those asymmetry centers that are present only in one potential structure (tautomer and protonation state) are not treated as stereogenic at the compound level. Similarly, some cis-trans bonds found in a structure might be missing from the corresponding compound.
Relations
The three structure levels have the following relations: compounds can have (can be represented by) multiple structures, and structures with well-defined, known configurations can have multiple conformers. Inversely, conformers have exactly one structure, and structures have exactly one compound.
Relation to products
Product is a special entry type that can appear on the mcule.com’s interface. It is connected to the chemical structures stored in the mcule database. Suppliers’ products are real, physical substances. Since these products can contain molecules in different tautomer and protonation states, their structural content can be best described at the compound level. The chemical content of products might be undetermined and in exceptional cases it can be changed, for example when supplier notices that the molecular structure was misdrawn or incorrectly determined previously.
In the mcule system only products with determined structures are registered. In the rare event of product structure correction, the corrected new structure is registered and will be associated with the product. Their structures are represented by compounds in the database: products with structures drawn in a different tautomeric form or protonation state are treated structurally equivalent.
Comparison to user structures/conformers
During the registration user structures/conformers (link) are kept intact. Structural equivalence is not detected, all entries are registered with an unique identifier in the user database. The only restriction is that user structures should have 2D coordinates whereas conformers should have 3D coordinates.
During calculations they are treated similar to structures and conformers of the mcule database. User structures are considered as being in that specific tautomer and protomer form represented in the 2D structure.