Table of Contents
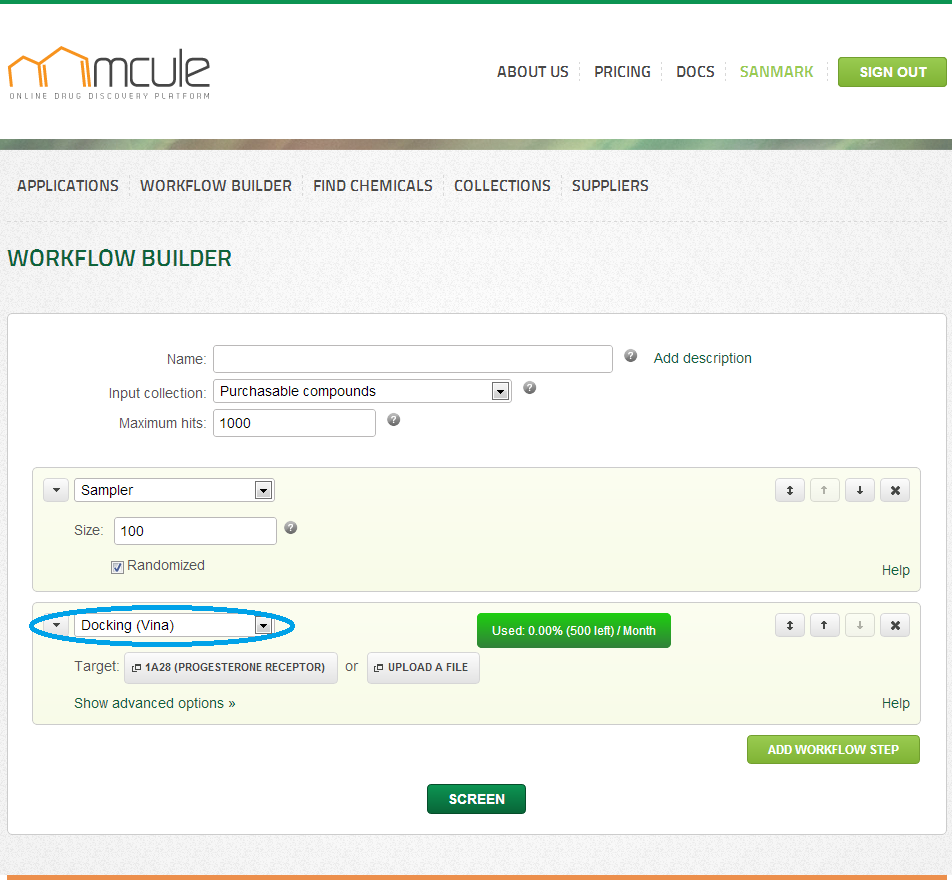
Docking (Vina)
Molecular docking is a method that predicts the binding orientation of one small molecule at the binding site of a target macromolecule (protein, DNA, carbohydrates, lipids, etc.) and estimates the binding affinity. Docking mainly consists of the following two steps: (i) pose generation, (ii) affinity prediction (scoring). During pose generation, the ligand is placed into the binding site by sampling its rotational and translational degrees of freedom. Subsequently, the affinity is estimated based on the generated pose. The Docking (Vina) workflow step utilizes the Vina docking algorithm, which has been developed by Oleg Trott et al. (Trott O, Olson AJ. J. Comput. Chem. 2010, 31, 455-61) and its robustness and high accuracy made it one of the most frequently used docking tools.
When to use
Docking plays a critical role in structure-based drug design projects, where the binding affinity of thousands or even millions of compounds should be assessed to identify potent inhibitors or activators of therapeutically relevant macromolecules, such as receptors, enzymes and ion channels. It can identify new drug candidates (structure-based virtual screening), or help to rationalize the binding mode of known ligands. It can give ideas about how ligands can be further developed to maximize interaction with the target. Docking can be utilized when the structure of the target has been experimentally determined or a high quality model is available.
How to use
If you are new to docking, it is suggested to choose one of the ~10,000 prepared target structures. If you are more experienced, you might upload your own target structure. In the latter case, you will need to specify the center of the binding site. For this, you can click on the “Select binding center” button. You will be able to select an atom in the opening target visualizer, which will be considered as the geometric center of the binding center.
Docking results are ranked by docking scores (the more negative the better), which indicates how well the ligand is predicted to bind to the target. It is, however, also important to check the binding mode and analyze whether it is in agreement with a priori knowledge.
Since docking is one of the most computationally intensive drug discovery tools, it is highly recommended to apply it in the last steps in a screening workflow. basic property filter, REOS filter and Diversity selection might be used to narrow down the search space to apply the Docking (Vina) step to the most interesting candidates only.
Options
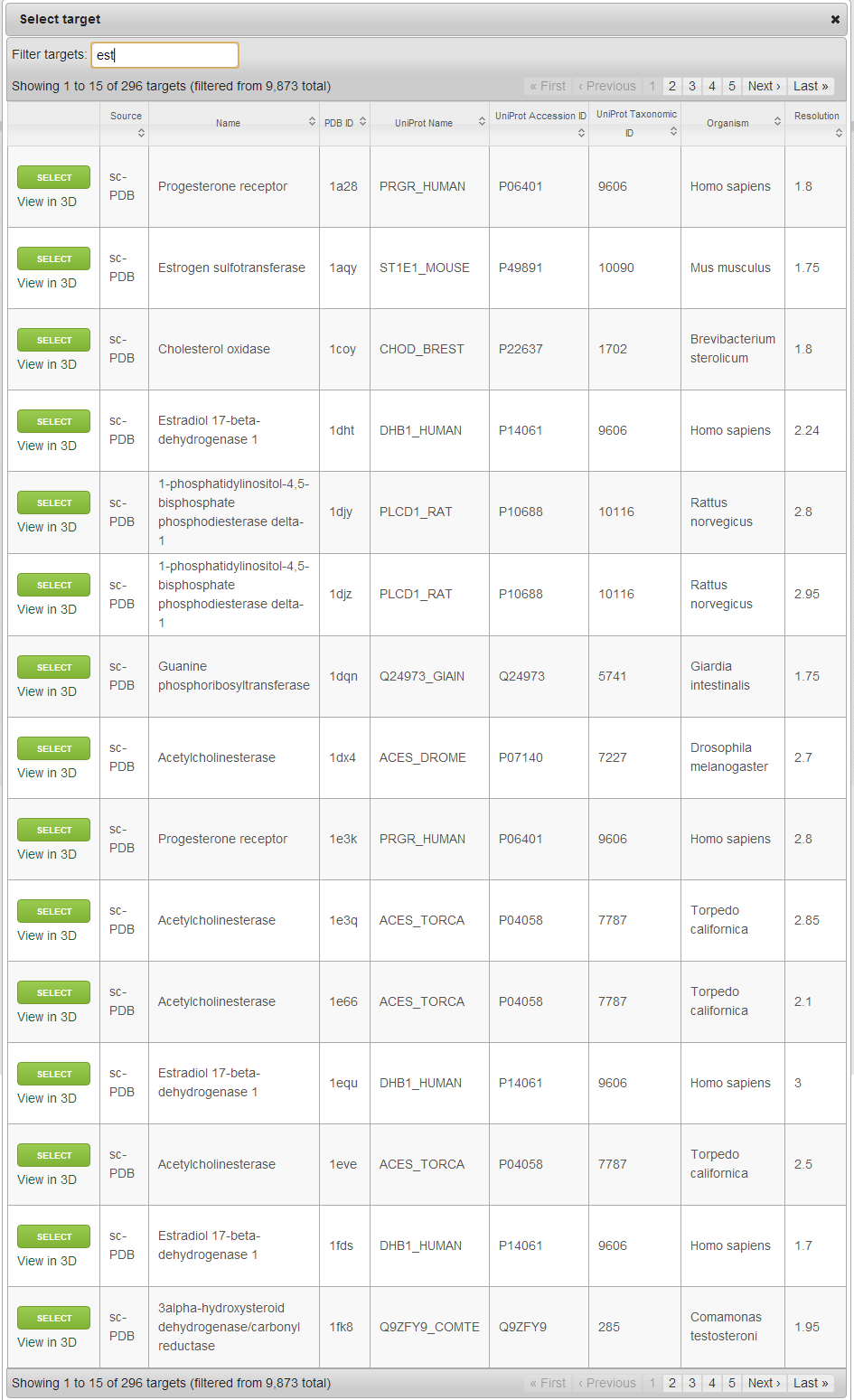
Nearly 10,000 automatically prepared target structures integrated from the sc-PDB database (Meslamani J, Rognan D, Kellenberger E. Bioinformatics. 2011, 27, 1324-6) are immediately for target selection. These and your previously uploaded targets are available by clicking on “Select target”. Alternatively, you can upload new targets in PDB, MOL2 or PDBQT formats by clicking on “Upload a file”.
Selecting target
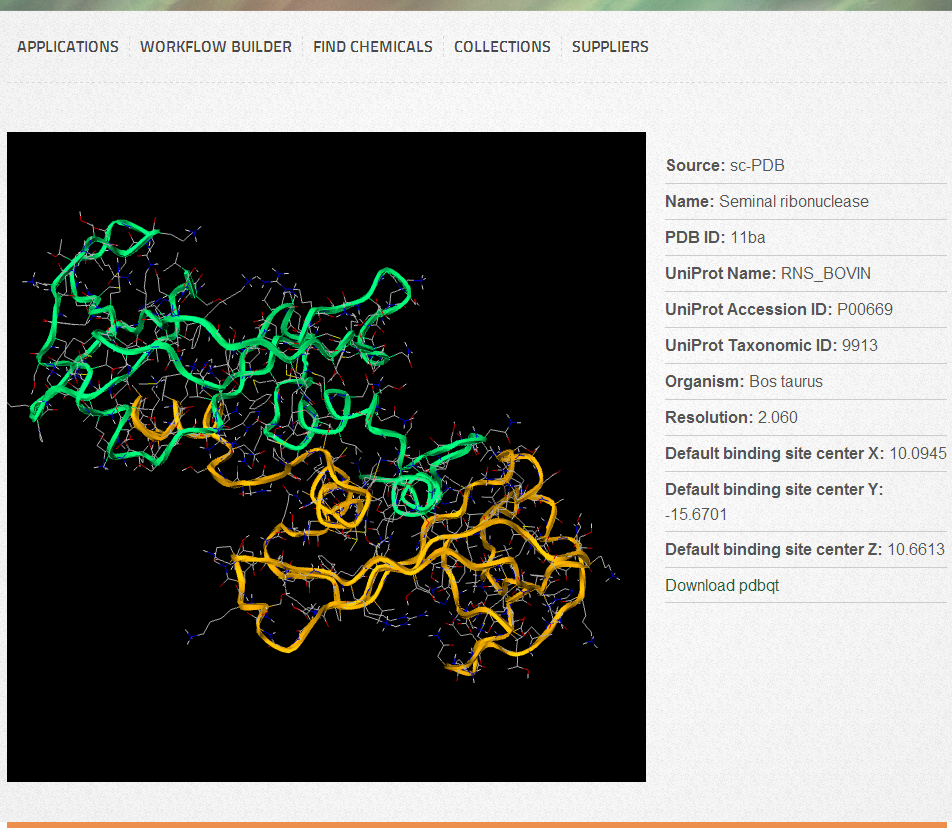
When browsing the already available targets, you can search/filter/rank the entries by PDB ID, Protein name, Organism name, UniProt Name/Accession ID/Taxonomic ID. Your previously uploaded targets will also appear here, but they can be deleted at any time (Click on “Delete” in the first column). Selection can be made by clicking on “Select” in the first column. To visualize the target, you can click on “View in 3D” under “Select”, which will open a new page with an interactive 3D visualization box containing the target.
Target visualization
By default, protein main chains are displayed as ribbons and sidechains as lines. Heteroatoms (if any) are displayed as sticks. You can use your mouse or touchpad buttons to rotate (Left button), zoom (Right button or Scroll or Shift+Left button), translate (Middle button or Ctrl+Left button) and slab (Ctrl+Right button). For 3D visualization we use the WebGL/Javascript based molecule viewer GLmol. If you experience any problems or your target is not displayed as it is shown on the Figure, you should verify that your browser supports WebGL, or you need to enable it manually. We suggest to use the latest version of Chrome or Firefox to get the highest level user experience.
Upload target
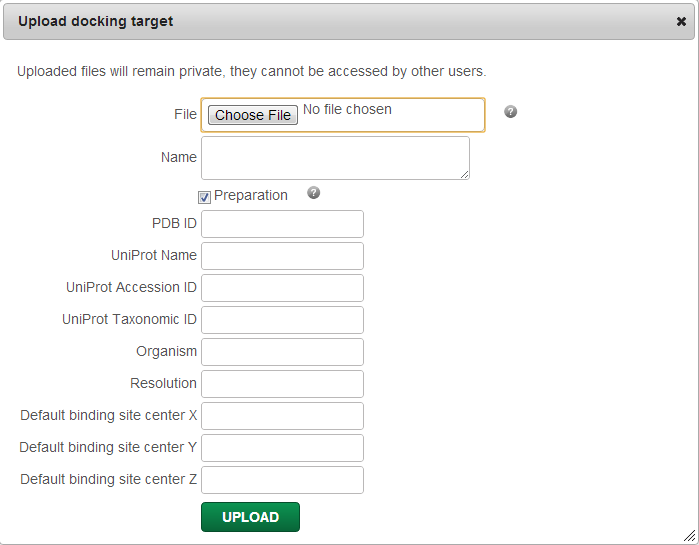
You can upload your own target by clicking on “Upload a file”. All uploaded files will remain private and won't be accessible by any other user.
For each uploaded file you can optionally add associated data, such as Name (name of the uploaded file by default), PDB ID, Uniprot Name, Uniprot Accession ID/Taxonomic ID, Organism, Resolution (RMSD of the experimentally determined target structure), and default X, Y, Z binding site coordinates. The given data will be stored and will be displayed and searchable in the “Select target” menu.
You can also run an automatic target preparation for docking by ticking the “Preparation” box, in which case, AutoDockTools will be utilized to add hydrogens on your target if none exists, add Gasteiger charges, merge charges and remove non-polar hydrogens, lone-pairs, water molecules and non-standard residues. If you opt out the automatic preparation, only a quick check for file validity will be performed.
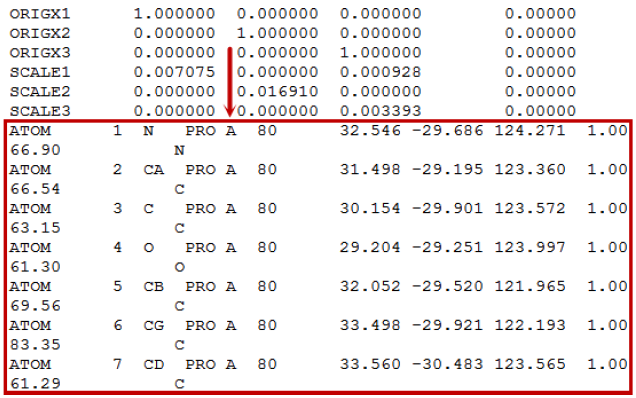
Please note that the PDB upload file size limit is currently 2MB. If you have a larger PDB file, we suggest to extract and upload only the binding site or a single monomer/chain from the PDB file. You can extract monomers by any text editors. To reduce the file size, you can eliminate all lines and keep the ATOM records only. You can find the chain identifier after the three letter residue name (in the below example “PRO” is the residue and “A” is the chain identifier).
Binding site center
X, Y and Z coordinates (from the coordinate system of the protein). These are automatically populated with values if they are present in the target database. Coordinates are available for all targets derived from the sc-PDB database. For alternative binding center selection, or for uploaded targets you can click on the “Select binding center” button, which will open the target in the target visualizer. You can pick any atom, which will be considered as the geometric center of the binding site.
Binding site area
Size of the binding size in Angstroms. Default value is 22, which typically covers a sufficiently large space for docking typical small molecules.
Maximum number of binding modes / ligand
Number of binding poses to be saved per ligand (default: 1, max: 9)
Exhaustiveness
This parameter sets the exhaustiveness of conformational sampling of docking (default: 2, max: 8). We found that a value of “2” is typically sufficient for the conformational sampling of small molecules.
Results
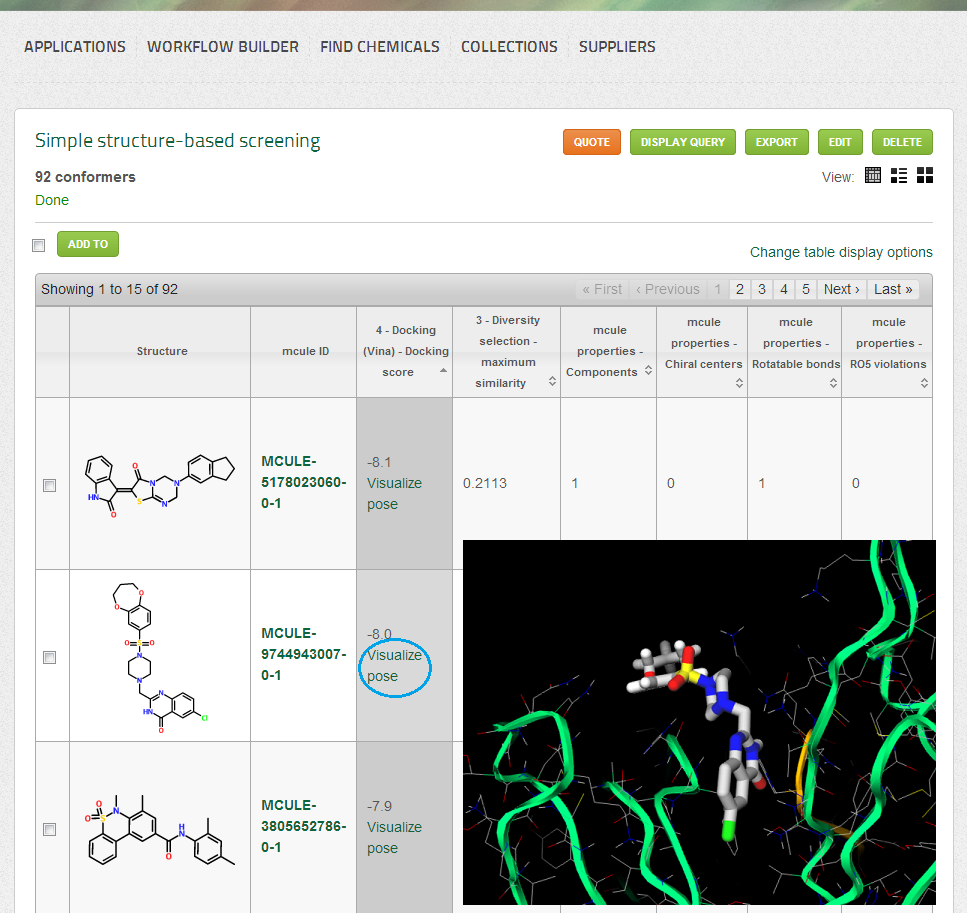
- Conformers for the successfully docked molecules
- Docking (Vina) score (displayed as a single column in both List and Table views): the docking score of Vina is a (very rough) estimation of the binding affinity (free energy of binding). More negative docking scores suggest higher affinity.
- Hits will be ordered by the Docking (Vina) score
- Docking poses can be visualized by clicking on the “Visualize pose” link under the docking score
Limits
The Docking (Vina) filter available in the Free package is limited to 10000 input molecules per month. If you need more, subscribe to our Docking (Vina) package.
Docking protocol
Docking (Vina) workflow step requires input ligands with a valid 3D structure, therefore the input ligand collection is prepared for docking as follows. Unknown or undefined tetrahedral stereocenters and cis-trans double bonds are converted into well-defined centers and double bonds by the stereoisomer generator of mcule. Molecules failed to dock are skipped. To ensure that molecule conversions did not affect the identity of the molecule, InChI strings of the docking input and output are compared and in case of InChI mismatch, the molecule is skipped.