Table of Contents
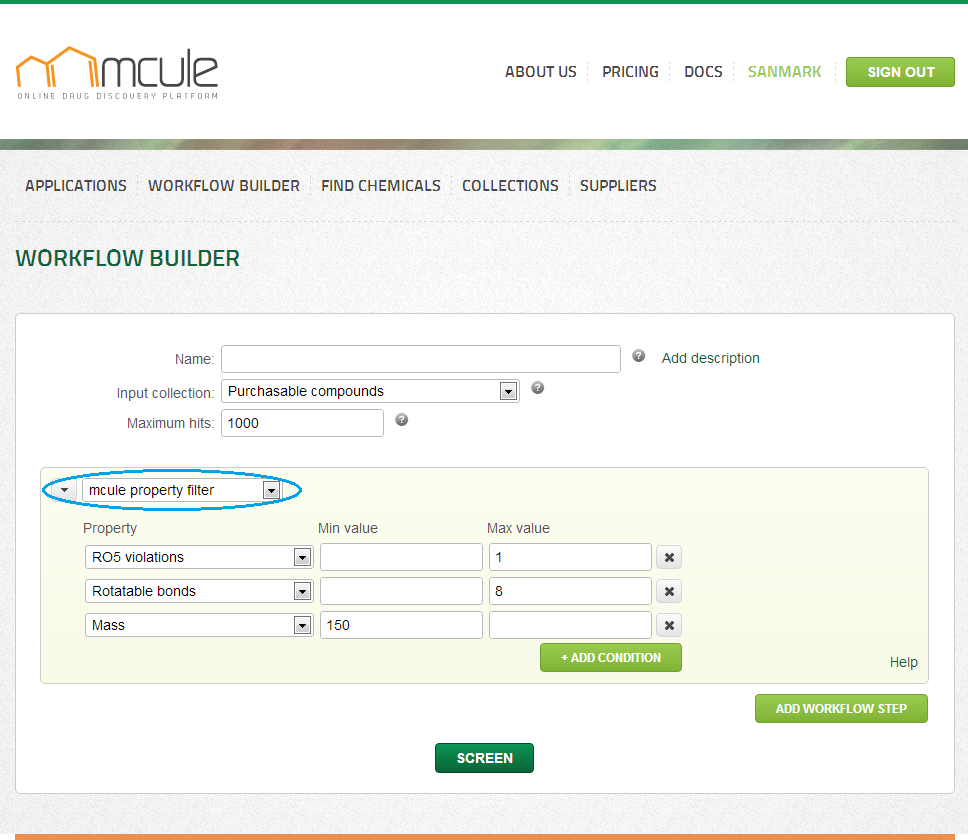
BASIC PROPERTY FILTER
With the mcule property filter, molecules can be filtered out based on their properties, such as Mass, logP, etc.
Physicochemical properties critically affect pharmacokinetic and pharmacodynamic properties of ligands. For example, high logP (> 5) and molecular weight (> 500 g/mol) are typically associated with unsuitable ADMET profile. Presence of adequately charged functional groups in the ligand can be critical for high-affinity binding. Careful selection of physicochemical properties is therefore crucial for the identification of hits suitable for further drug development.
When to use
It is recommended to use property filtering in each screening workflow to narrow down the search space for the most relevant molecules. For general small-molecule drug discovery projects where the goal is to develop an orally active agent, the number of rule-of-five (RO5) violations should be kept at minimum.
How to use
It is recommended to adjust the mcule property filter to the actual project. For example boundaries might be defined based on the properties of known reference ligands acting on the target. If all reference ligands contain an H-bond donor functionality, it might make sense to set a minimum 1 H-bond donor limit. Fragment-based drug discovery projects require more strict limits in terms of molecular size and lipophilicity. It is therefore recommended to use rule-of-three (RO3) violation filter for such projects.
List of available properties
Results
- Molecules satisfying search criteria
- All properties included in the mcule property filter will be displayed in List and Table views
- Any other mcule properties can be displayed in Table view by clicking on the “Change table display options” link
Access
Mcule properties are provided in the Free package.