Table of Contents
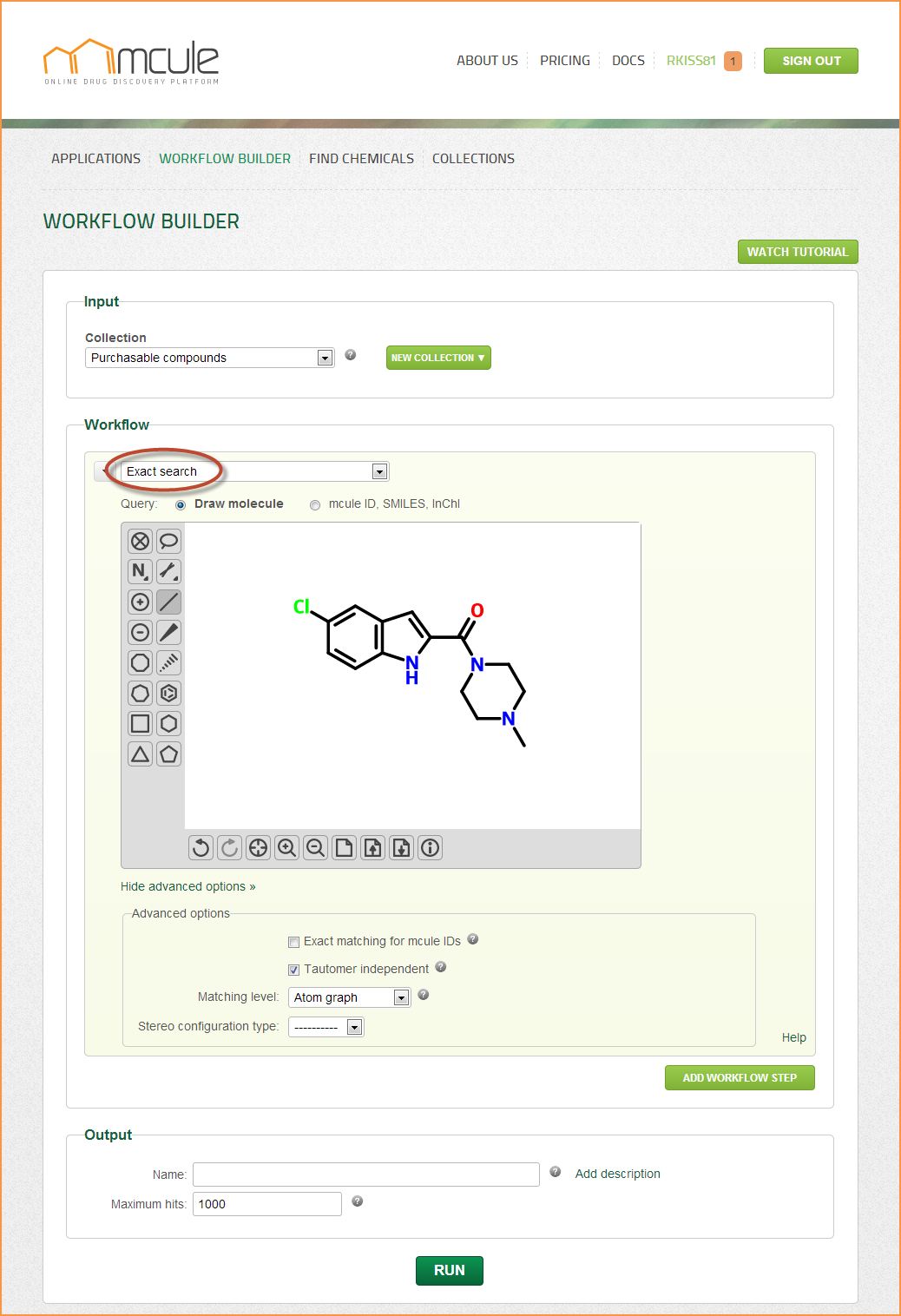
Exact search
The primary goal of exact searching is to identify one particular molecule or a set of isomers of the same molecule.
When to use
Exact search can be useful when checking commercial availability of a specific molecule, e.g. a reference ligand. You can also check whether a collection contains a specific molecule or not.
How to use
Exact searching in mcule is based on InChI. The layered structure of the InChI string allows to make the comparison of the query and the target on different matching levels. Users can choose from the following “Matching level” options: Full, Sp3 configuration, Sp2 configuration, Atom graph, Heavy atom, Formula.
- Full (tautomer dependent):
- InChI=1/C11H11ClFNO2/c1-6(15)9-4-7(11(14)16)2-3-8(9)10(13)5-12/h2-6,15H,1H3,(H2,14,16)/b10-5-/t6-/m0/s1/i1+2/f/h14H2
- Full (tautomer independent):
- InChI=1/C11H11ClFNO2/c1-6(15)9-4-7(11(14)16)2-3-8(9)10(13)5-12/h2-6,15H,1H3,(H2,14,16)/b10-5-/t6-/m0/s1/i1+2
- Sp3:
- InChI=1/C11H11ClFNO2/c1-6(15)9-4-7(11(14)16)2-3-8(9)10(13)5-12/h2-6,15H,1H3,(H2,14,16)/b10-5-/t6-/m0/s1
- Sp2:
- InChI=1/C11H11ClFNO2/c1-6(15)9-4-7(11(14)16)2-3-8(9)10(13)5-12/h2-6,15H,1H3,(H2,14,16)/b10-5-
- Atom graph:
- InChI=1/C11H11ClFNO2/c1-6(15)9-4-7(11(14)16)2-3-8(9)10(13)5-12/h2-6,15H,1H3,(H2,14,16)
- Heavy atom:
- InChI=1/C11H11ClFNO2/c1-6(15)9-4-7(11(14)16)2-3-8(9)10(13)5-12
- Formula:
- InChI=1/C11H11ClFNO2
It is also possible to set whether the exact search should be performed on the specific tautomer (and protonation state) specified in the input, or the search should be tautomer (and protonation state) independent (“Tautomer independent” box).
Accepted stereo configuration type of the target molecules can be specified in the “Stereo type” field. The stereo configuration type of a molecule can be “None” (the molecule does not have any stereocenters), “Unknown” (it is not known whether the stereo configuration is absolute or racemic or relative), “Absolute”, “Relative” and “Racemic”. If this field is left blank, the search will retrieve molecules with any stereo type. To learn more about the meaning of the different stereo types, check our stereochemical notations.
Queries can be drawn by the molecule sketcher, which can be hidden and reopened by clicking on the “Hide/Show sketcher” link. Queries can be also defined by entering mcule IDs, InChI or SMILES strings into the input field. 2D representations can be generated by clicking on the “Generate 2D” button. Note: If the “Generate 2D” button was clicked, the search will be performed on the generated 2D representation (2D SDF exported from the sketcher). If, however, the “Generate 2D” button was not clicked, the search will be directly performed on the mcule ID, InChI or SMILES strings coming from the input field. The results might be slightly different in these two cases.
Results
Molecules satisfying search criteria